Entos Pharmaceuticals
An Edmonton based Pharmaceutical company is advancing to human trials for Covid-19 vaccine.
Entos Pharmaceuticals is a healthcare biotechnology startup company led John D. Lewis, the Frank and Carla Sojonly Chair at the University of Alberta.
Yesterday, Entos released a statement saying that they have selected two lead candidates for a DNA-based vaccine.
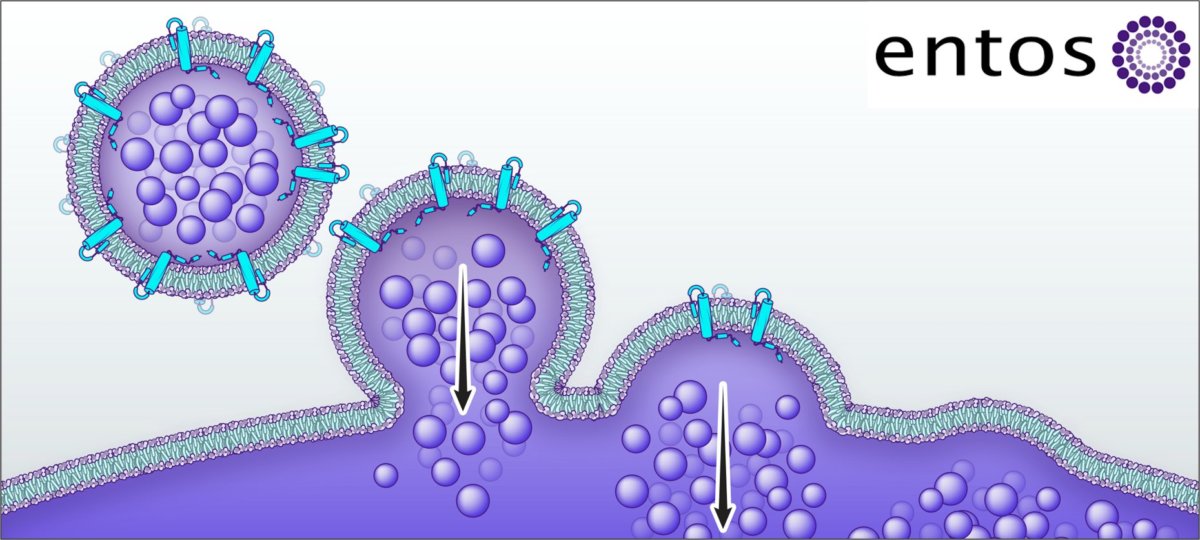
Entos had launched the Fusogenix drug delivery platform at the onset of the Covid-19 pandemic.
The program is based on a DNA vaccine approach rather than the traditional vaccines.
The traditional vaccine works by introducing a virus or a part of it into the body which triggers an immune response. On the other hand, DNA vaccine uses a piece of viral DNA to immunize the body. This approach is thought to be more effective and advantageous against pan-Coronavirus infection in the long term.
“Based on the preclinical in vivo safety and efficacy data, we believe our Fusogenix DNA vaccine candidates have the potential to be safe and highly potent vaccines that will provide protection against COVID-19 as well as future coronavirus threats,” said John Lewis, CEO of Entos Pharmaceuticals.
As per the release, Entos’ leads, based on the Fusogenix platform are encoded with optimized Covid-19 spike fragments. The selection was based on robust preclinical studies including potency, high immunogenicity (ability to induce an immune response) and efficacy as well as meeting the ADE safety assessment standard (ADE is minimum drug dose that can be administered without adverse effects).
Entos has been granted a $4.2M grant from the Canadian Institutes of Health Research as part of its rapid research funding competition.
The money will be spent on further development of vaccine candidates.
They will partner with the Canadian Center for Vaccinology and Dalhousie University in Nova Scotia to initiate Phase I/II human clinical trials by late July.
Phase 3, which would involve working with larger groups of people, could potentially start by the end of 2020.
If all goes well, Entos aims to develop a safe and effective Covigenix DNA vaccine for COVID-19 in one year.
More Information is available here.








How can a person sign up for the Phase III trials