Credit: Medicago
A Phase 2 trial of Canada’s first homegrown COVID-19 vaccine is showing promising antibody results.
Medicago executive vice-president Nathalie Landry says the Quebec-based company’s vaccine produced 10 times the antibodies in adults compared with those who have had COVID-19 during the phase 2 trial.
The results are not yet peer-reviewed but Landry says her team is “quietly confident” the vaccine will also prove to be very effective at preventing COVID-19 infections.
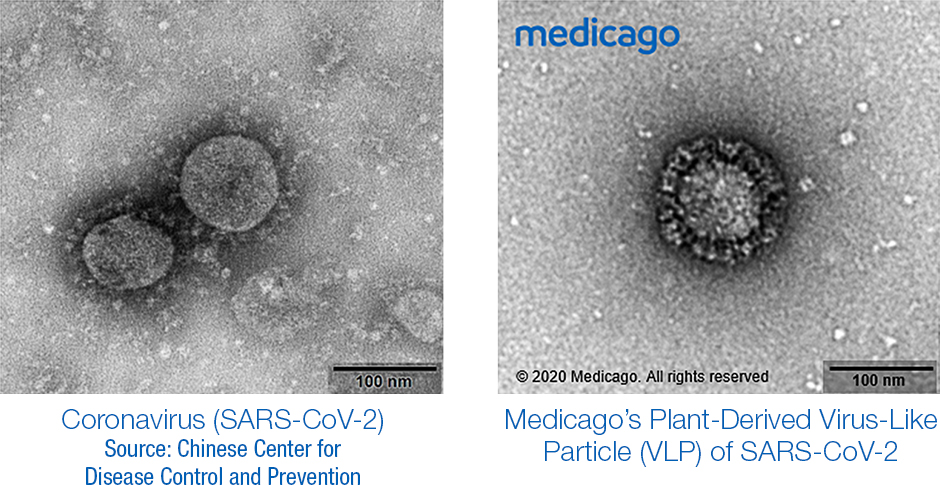
Medicago’s vaccine uses a cousin of the tobacco plant to grow a particle that resembles the virus that causes COVID-19 but contains none of the genetic material.
According to the press release, the Phase 2/3 study is ongoing and it is a multi-portion design to confirm that the chosen formulation and dosing regimen of CoVLP (two doses of 3.75 µg CoVLP combined with GSK’s pandemic adjuvant given 21 days apart) has an acceptable immunogenicity and safety profile in healthy adults 18-64 years of age, elderly subjects aged 65 and over and adults with comorbidities.
The Phase 3 trial of the vaccine candidate launched on March 16, 2021.
Trial sites are currently enrolling subjects in Canada, the United States, the United Kingdom, and Brazil, with additional sites expected to be added in the coming weeks. The vaccine candidate has received Fast Track designation by the FDA in the United States, and Health Canada has initiated a review of Medicago’s COVID-19 rolling submission under the Interim Order.
Canada pre-purchased 20-million doses of Medicago’s vaccine but most Canadians will be vaccinated before the shot is approved.
— With Files From The Canadian Press








There a typo in your text.
The Medicago trial should be on March 15 2022 (not 2021)
I don’t care if it’s made by vegans using vegan non leather needles in a grass hut…they’re still injecting foreign chemicals into a body I worked hard to detox and rid of anything non plant based.